Nanotechnology offers, for the first time, tools to develop new industries based on cost-effective and cost-efficient economies, thus seriously contributing to sustainable economic growth. The areas where nanotechnologies work for sustainable electricity storage basically are rechargeable batteries and supercapacitors [1].
1. Nanoparticles for lithium-ion (Li-ion) rechargeable batteries Li-ion batteries exhibit the merit of long cycle life, high electrochemical capacity, high energy density and no memory effect unlike lead acid and Ni-Cd batteries [2]. A typical rechargeable Li-ion battery contains a positive electrode (cathode), a negative electrode (anode) and an electrolyte-filled separator with permselectivity for the Li-ion. Commercially, the anode is composed of a carbon material (graphite) and the cathode is constructed by the lithium metal oxide with a much more positive redox potential, and a Li-ion conducting electrolyte is filled between the cathode and anode as a separator [3]. Common reactions that involve a rechargeable Li-ion battery are show in Fig. 1.
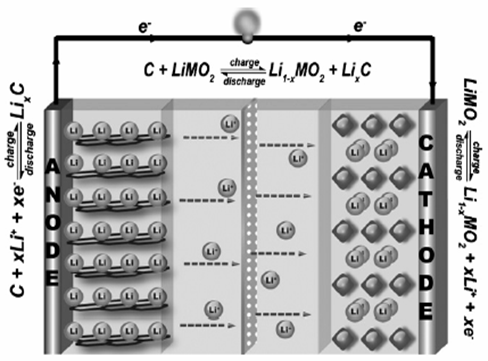
Fig. 1 Typical rechargeable Li-ion battery scheme and the chemical reactions involved. Adapted from Ref. [2]. The deposition of crystalline molybdenum by the hot-wire chemical vapor deposition (HWCVD) has been employed as an economically scalable method for the deposition of crystalline molybdenum oxide nanoparticles at high density and exhibiting a high rate capability as anticipated for the reduced solid-state Li-ion diffusion length [4]. The synthesis of carbon-encapsulated metal oxide hollow nanoparticles (HNPs) and pure metal oxide HNPs, has been developed from carbon-encapsulated metal nanoparticles by controlled oxidation in air via the nanoscale Kirkendall effect. The most important result from electrochemical measurements indicated that there structures exhibit excellent cycling performance and a high reversible capacity of about 700 mAh/g up to 60 cycles, when they were used as the anode materials for lithium-ion batteries [5]. LiMn2O4 nanoparticles are synthesized by a novel sol–gel thermolysis process using polyvinyl alcohol with three different combustion fuels such as urea, hexamine and citric acid to determine their effect on structure, particle size and electrochemical properties of LiMn2O4 nanoparticles. PVA-urea results showed the effective combination in sol–gel thermolysis process for the synthesis of cubic spinel powder and the compound obtained by using this combination is an effective cathode active material for Li-ion battery applications [6]. Recently, lithium-ion battery research has focused on the development of high-energy cathodes based on new organosulfurbased materials. These compounds with multiple thiol groups or disulfide moieties (see Fig. 2) have received attention as cathode active materials for lithium/lithium-ion rechargeable batteries due to their high theoretical capacities. In addition, the capability of the thiolate redox chemistry to release and capture lithium ions during discharge enable them to be easily incorporated into a ‘‘rocking-chair’’ type system employed in Li-ion batteries [7].
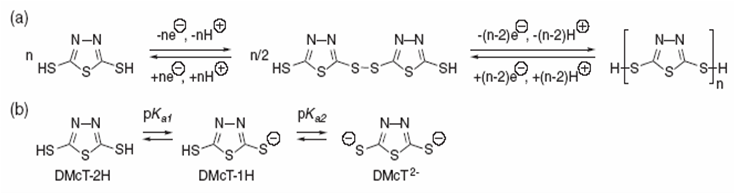
Fig. 2 Redox reactions and proton equilibria for 2,5-dimercapto-1,3,4-thiadiazole. Adapted from Ref. [7]. 2. Nanoparticles for electrodes to use them in supercapacitors A supercapacitor is an electrical capacitor that consists of two polarizable electrodes that store electrical energy [1, 3]. So called electrochemical capacitors, supercapacitors possess much higher power densities (> 500 W/kg), excellent reversibility, and longer cycle life (> 105 cycles) compared with rechargeable batteries [8]. Recent studies show the potential applications of carbon nanotubes (CNTs) as electrode materials in supercapacitors [9]. Conventional carbon nanotubes are made of seamless cylinders of hexagonal carbon networks and are synthesized as single-wall (SWCNT) or multiwall carbon nanotubes (MWCNT). Carbon nanostructures possess metallic or semiconductor properties that can induce catalysis by participating directly in the charge transfer process [10]. Hsieh et al. [11] reported the electrochemical behavior of polyacrylonitrile (PAN)-based carbon fabric decorated with carbon nanotubes, and examined by cyclic voltammetry and charge-discharge cycling the electrochemical behavior of the carbon electrodes in H2SO4. These studies indicated that presence of nanotubes, generated decreasing capacitance effect and inner resistance, but enhance the double-layer capacitance, which has created a great enhancement on development of electrochemical capacitor. Specific capacitance calculations in nano ZnO-activated carbon (AC) composite electrodes reveled that the composite electrode with 1:1 (ZnO:AC) composition has higher specific capacitance.
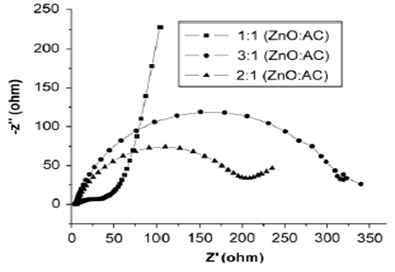
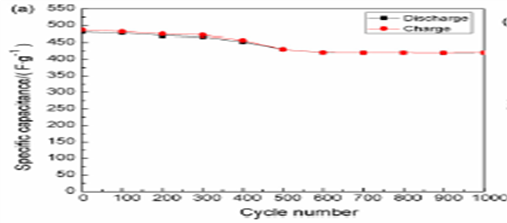
Fig. 3 Nyquist’s plots for ZnO-AC composites at compositions of 1:1, 2:1 and 3:1. Adapted from Ref. [12]. Fig. 3 shows the Nyquist plot for electrodes with three different compositions. The graph with 1:1 composition exhibits lowest resistance and high capacitance. The specific capacitance of the electrodes is reduced with increase in zinc oxide content. Chen et al. [13] reported that Ni3(Fe(CN)6)2(H2O) electrode exhibited good capacity retention and capacitive property. The variation of specific capacitance as a function of cycle number at the current density of 1.4A/g is showed in Fig. 4.