A catalyst is a substance which increases the rate of reaction without being consumed appreciably during a reaction process. This write up is focused on Heterogeneous catalysts, which promote the conversion of the reactants, and that are on a different phase than reactants and products. Normally, heterogeneous catalysts are found in solid phase or they are anchored to porous surfaces. Figure 1 illustrates structure of covalent, traizine-based network (CFT) with Platinum catalyst of researchers from Max Planck Institute for Coal research and Max Planck Institute of Colloids and Interfaces. One of their objective is to convert methane natural gas to methanol at 215 ?C and has approximately 75% for favorable conversion.
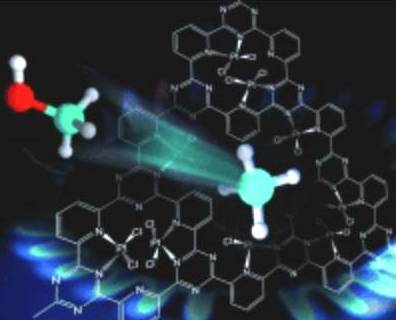
Figure 1: Methane to methanol solid surface catalyst illustration from Max Planck Institute of Coal research and Max Planck institute of Colloids and Interfaces, (See New Energy and Fuel)
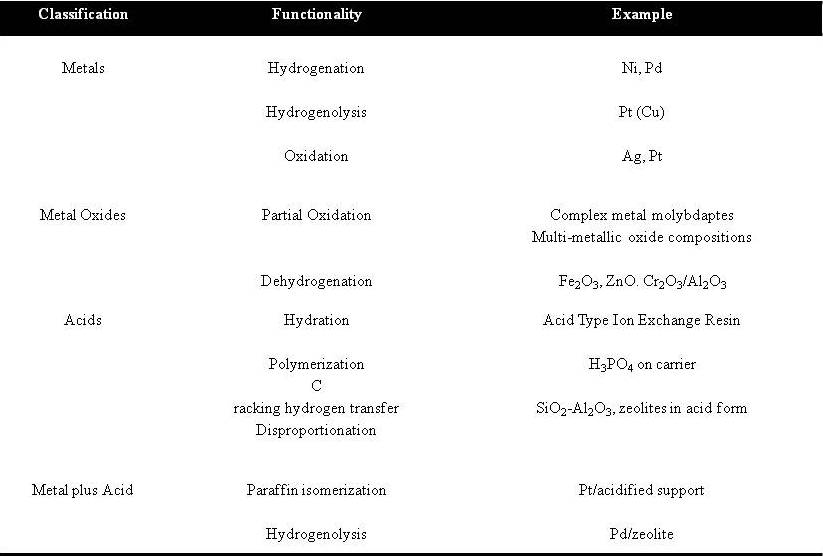
Generally, the heterogeneous catalysts are classified according to the type of functionality that they show during the reaction. Table 1 shows the general classification for the heterogeneous catalysts from the book ‘Heterogeneous Catalysis in Practice’ by Satterfield on the 1980’s.
Table 1: General classification of heterogeneous catalyst by its’ functionality during a reactionTaken from page 20 of Heterogeneous catalyst in Practice, Satterfield 1980
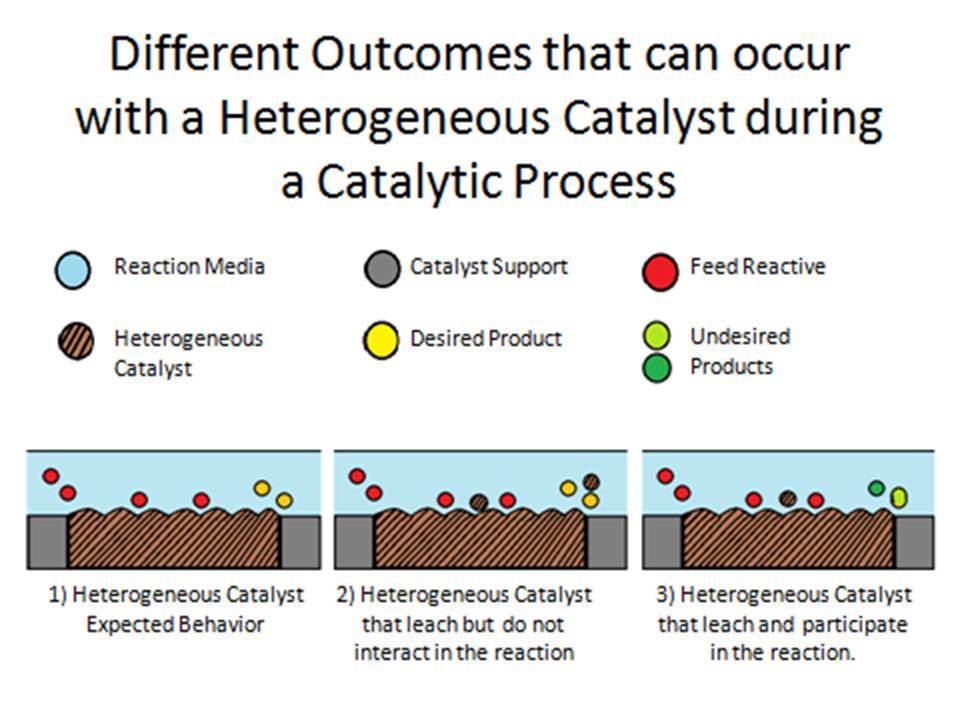
According to the literature, heterogeneous catalysts could be composed of metals, parts or molecules that:, 1) don’t leach and these catalysts are considered truly heterogeneous, 2) leach and do not interact in the reaction process, and 3) leach and participate in the reaction process but as homogeneous catalyst Pinel, 2009. Figure 2 shows this behavior where an assumed porous catalytic bed material behavior is presented on the three scenarios.
Figure 2: Different outcomes occurring that can occur with a heterogeneous catalyst during a catalytic process
In order to better understand those catalytic structures, properties, and functionalities, powerful instrumentation has been developed. Some of this instrumentation is presented below.
Table 2: Summary of general techniques used for characterize heterogeneous catalysts. More information could be obtained from Zhen Ma, Surface and Nanomolecular Catalysis, 2006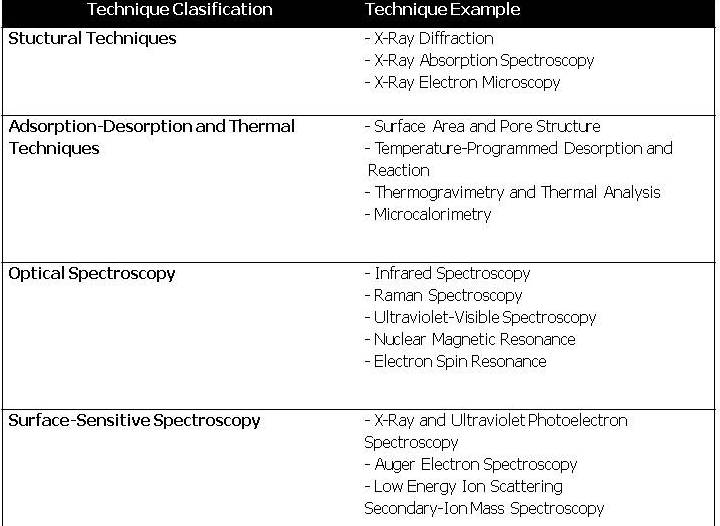
It is worth to recognize that thanks to these instruments, catalytic properties, reaction mechanism and surface interaction have been revealed for many reaction systems. Recently, our necessity to predict and understand the functionality, structure role, geometry, and ligand-group effect of the heterogeneous catalyst beyond the surface has sparked the development of computational chemistry methods based on quantum mechanical (QM) calculations. One of the objectives of using QM calculations is to understand heterogeneous catalyst mechanistic behavior by means of understanding the molecular interactions. Calculations of properties like catalyst geometry, and electron density can be obtained with different methods as illustrated on Figure 3. However, finding a method that can accurately represent an heterogeneous catalytic system could represent a challenge. An example of this is presented in the Figure 3 from an article written by Jeanet Conradie and Abhik Ghosh on 2007, ‘DFT Calculations on the Spin-Crossover Complex Fe(salen)(NO): A quest for the Best Functional’. Where by the use of different functionals for the Density Functional Theory (DFT) calculation method and same conformation of Fe(salen )(NO) they obtain different spin density profile.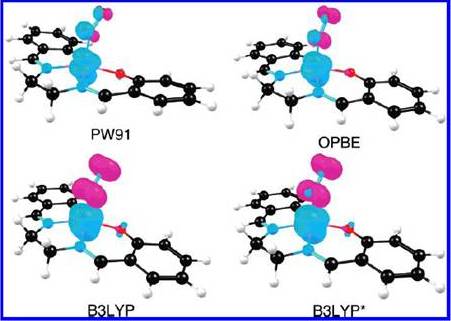
Figure 3: Spin Density profiles obtained by change of the functional using DFT calculation method. Complete study is available on Jeanet Conradie and Abhik Ghosh Phys.Chem. B, 2007
With the appropriate method, the catalytic and molecular behavior can be studied with comparable results to analytical techniques, such as spectroscopic methods shown in the Figure 4. Where studies like low energy electron diffraction (LEED), sum frequency generation (SFG), and Temperature Programmed Desorption (TPD) show concordance with a simulation model for the hydrogenation of ethylene on Pt(111) Ma and Zaera et al, where data suggest the formation of ethylidyne on the catalyst surface during the reaction. This is an example that illustrates how simulations can help us to understand interaction between molecules and the catalytic surface of a heterogeneous catalyst.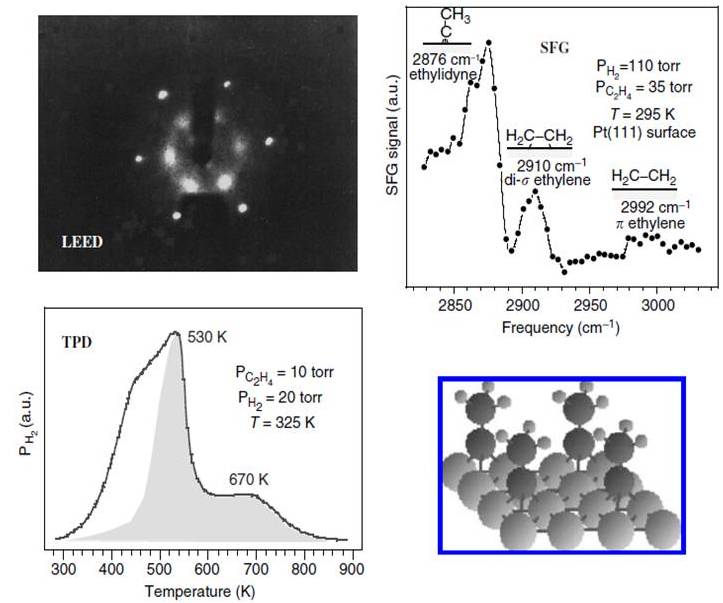
Figure 4: Concordance between instrumental techniques and catalyst model for hydrogenation of ethylene on a Pt (111) surface shown on Zhen Ma and Francisco Zaera on Surface and Nanomolecular Catalysis, 2006
References:
A Method to Get Natural Gas to Transportable Methanol. New Energy and Fuel: News and Views for making and saving money in new energy and fuel; Nov, 24, 2009,http://newenergyandfuel.com/http:/newenergyandfuel/com/2009/11/24/a-method-to-get-natural-gas-to-transportable-methanol/ , (Accessed: March 27, 2010).
Conradie, J.; Ghosh, A. DFT Calculations on the Spin-Crossover Complex Fe(salen)(NO): A quest for the Best Functional. J. Phys.Chem. B 2007, 111, pp. 12621-12624.
Ma, Z.; Zaera, F. Characterization of Heterogeneous Catalysts. Surface and Nanomolecular Catalysis; Richards, R. Ed; CRC Press, 2006; pp. 2-32.
Pinel, C.; Noël, S.; Pan, K.; Luo, C.; Djakovitch, L. Synthesis of New Ligands for the Preparation of Combined of the Homogeneous and Heterogeneous Catalysts. Catalysis of Organic Reaction 22 Conference; Prunier, M. L. Ed; CRC Press, 2009; pp. 185-192.
Satterfield; C. N.; Brown, J. V. Introduction and Basic Concepts. Heterogeneous Catalyst in Practice; Golden, S., Amerman, S. Eds.; McGraw-Hill: New York, 1980; pp. 1-22.