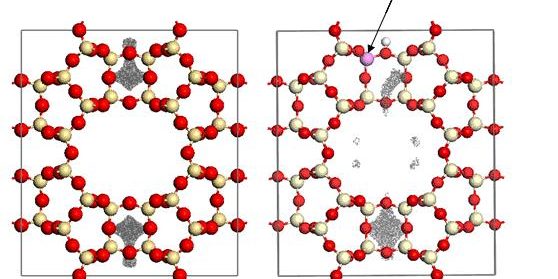
Javier A. Huertas-Miranda and María M. Martínez-Iñesta Through our molecular simulation studies related to the templated synthesis of metal nanowires, we have found that the presence of Al in the zeolite framework has an important influence on the positioning of the guest metal atoms. This is especially important for the MOR framework, which has side pockets that can be accessed through interconnecting rings. In Metropolis Monte Carlo (MMC) simulations with the CAN framework with Si/Al = ? and Si/Al = 1, it was observed that the presence of Al in the zeolite structure promoted a separation of the Ni neutral atoms from the wall surfaces of the zeolite structure. To study this behavior in detail, we performed MMC simulations with one Ni atom in the MOR framework with Si/Al = ? and with Si/Al = 47.[1] In the MMC simulation for MOR with no Al, the Ni atom was positioned at the side pockets of the structure with a symmetrical trace of the Ni atom (see the figure above). It is evident that Ni was located at relatively short distances from the side pocket walls. For the case of MOR with one Al atom at the T3 site (Si/Al=47), the trace of the Ni atom was distanced from the wall surface atoms of the site pocket where the Al atom was positioned. The trace of the Ni atom was also more evident in the main pore channel, suggesting that the increase in the repulsion forces at the wall surface near the Al atom promotes the displacement of Ni to the main pore. This behavior suggest that the Van der Waals (VdW) repulsion forces between the metal atom and the oxygen atoms bonded to Al atoms is stronger than the repulsion forces from Ni and the oxygen atoms bonded to Si atoms. The magnitude of the VdW repulsion forces is usually associated with the interaction of the electron cores of the nearby atoms. This possible effect over repulsion forces will ultimately by reflected in the parameterization of the pcff forcefield used in our studies.
Lunes a Viernes 7:45AM - 11:45AM y 1:00PM - 4:30PM(787) 832-4040 Ext. 2568 or 2587inqu.rum@upr.edu
Institucional
Effect of Al in the Positioning of Guest Metal Atoms in Zeolites
You are here:
- Home
- Effect of Al in the…